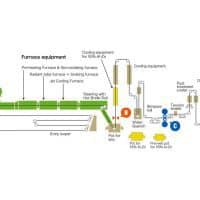
Hot-dip galvanizing is the process of passing steel through zinc pot to produce a corrosion resistant coating. Once coated, the steel runs through a set of air knives which blow away the excess coating. As a result, a coating forms on all surfaces, and attains a uniform thickness throughout the part.
While there are many different ways steel can be galvanized, the process is inherently simple, and includes three general steps: surface preparation, galvanizing, and post-treatment.
Surface preparation:
The purpose of surface preparation is to clean the steel by removing all oxides and contaminating residuals. This is done because zinc will not react with unclean steel.
Cleaning and preparing the steel to be galvanized consists of three steps:
- Degreasing/Caustic Cleaning. The steel is submerged in an acid bath that removes any dirt or grease off the steel. Then, the steel is rinsed with water.
- Pickling. During this stage, the steel is put in a diluted solution of either hydrochloric or sulfuric acid in order to remove any oxides and mill scale. After pickling, the steel is rinsed once more with water.
- Fluxing. The steel is dipped in the flux, which removes any oxidation that developed since pickling, and also creates a protective layer that prevents additional oxidation before the galvanizing process.
Once the steel is prepared, its surface is ready to be galvanized.
Galvanizing:
During this stage, the steel is passed through a zinc pot, which follows the ASTM B6 requirements. The pot(called a galvanizing kettle) is heated to temperatures ranging from 820-860 Fahrenheit, at which point the zinc is in a liquid form. Then, the steel is lowered into the bath, where zinc diffuses to the steel. Once the diffusion of zinc and iron is completed, the steel is withdrawn from the bath.
Post-Treatment:
When the steel is removed from the galvanizing kettle, it might undergo additional treatment to enhance its coating and protect the galvanized steel during transport and storage.